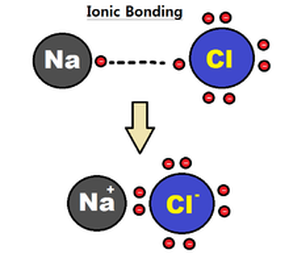
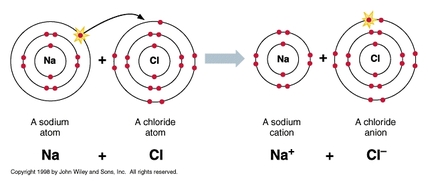
Ionic Bonds
The attraction between two oppositely charged ions. Ionic bonds form as a result of the attraction between positive and negative ions. For example, sodium loses an electron and becomes a positively charged ion. Chlorine gains an electron and becomes a negatively charged ion. They are attracted to one another.